|
Background: |
The carboxy terminus of Hsc70-interacting protein (CHIP, STUB1) is a co-chaperone protein and functional E3 ubiquitin ligase that links the polypeptide binding activity of Hsp70 to the ubiquitin proteasome system. Cytoplasmic CHIP protein contains three 34-amino acid TPR (tetratricopeptide repeat) domains at its amino terminus and a carboxy-terminal U-box domain. CHIP interacts with the molecular chaperones Hsc70-Hsp70 and Hsp90 through its TPR domain, while E3 ubiquitin ligase activity is confined to the U-box domain. The binding of CHIP to Hsp70 can stall the folding of Hsp70 client proteins and concomitantly facilitate the U-box dependent ubiquitination of Hsp70-bound substrates. CHIP appears to play a central role in cell stress protection and is responsible for the degradation of disease-related proteins that include cystic fibrosis transmembrane conductance regulator, p53, huntingtin and Ataxin-3, Tau protein, and α-synuclein. |
|
Applications: |
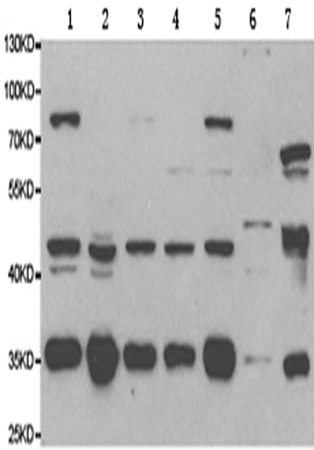
WB |
|
Name of antibody: |
STUB1 |
|
Immunogen: |
Fusion protein of human STUB1 |
|
Full name: |
STIP1 homology and U-box containing protein 1, E3 ubiquitin protein ligase |
|
Synonyms: |
STUB1; CHIP; HSPABP2; NY-CO-7; SDCCAG7; UBOX1 |
|
SwissProt: |
Q9UNE7 |
|
WB Predicted band size: |
35 kDa |
|
WB Positive control: |
HeLa, PC12, COS1, COS7 and OVCAR3 cells, muscle and liver tissue |
|
WB Recommended dilution: |
500-2000 |

 购物车
购物车 帮助
帮助
 021-54845833/15800441009
021-54845833/15800441009